Inflammatory bowel disease (IBD) is a term that encompasses two conditions - Crohn’s disease and Ulcerative colitis, characterized by chronic inflammation of the gastrointestinal tract. Prolonged inflammation results in damage to the GI tract. Inflammatory bowel disease in recent times is causing a significant healthcare burden as both and Crohn's disease and ulcerative colitis require lifelong therapy and constant monitoring.
Although the disease severity is mild, the risk for colorectal cancer is high. The incidence of paediatric IBD is on the rise. The major burden of IBD in India at present is in children, adolescents and teens. Men appear to be more frequently affected than women.
Symptoms
- Persistent diarrhea.
- Abdominal pain.
- Rectal bleeding/bloody stools.
- Weight loss.
- Fatigue.
Diagnosis
It is critical to diagnose IBD accurately and to distinguish it from irritable bowel syndrome (IBS). IBS is a functional bowel disorder characterized by group of symptoms like frequent bouts of bowel disturbance – diarrhoea or constipation, abdominal pain, discomfort, and bloating. There is no clear cause, no distinctive pathology and treatment is symptomatic. Exacerbations may be triggered by diet or stress.
According to the World Gastroenterology Organization, the diagnosis of IBD relies on a combination of patient’s history, physical examination as well as a few diagnostic tests including endoscopy (for Crohn’s disease) or colonoscopy (for ulcerative colitis), biopsy, imaging studies such as CT, MRI with contrast, blood and stool (Faeces) tests
Endoscopy is the gold standard method for the diagnosis of IBD. Endoscopic procedures, are however unpleasant, painful, time-consuming, requires bowel cleansing for proper visualisation, which may require hospitalisation.
Calprotectin
Calprotectin is a protein found in neutrophils, and, to a lesser extent, in monocytes and macrophages. It is found in biological material throughout the body mainly in plasma, urine, cerebrospinal fluid, faeces, saliva or synovial fluid. Calprotectin plays a major role during inflammation and is a positive acute phase protein.
Faecal calprotectin is a very sensitive, reliable, simple, cost effective and non-invasive bio marker for inflammatory bowel disease. The fact that faeces is in direct contact with the intestinal mucosa makes estimation of calprotectin level in faeces, an ideal, precise marker for detection of IBD. Therefore, due to its specificity for gastrointestinal tract inflammation, faecal calprotectin is superior to serum calprotectin. It is homogenously distributed in stools and stable in stool for up to one week at room temperature.
Faecal calprotectin concentrations demonstrate good correlation with severity of intestinal inflammation.
Faecal calprotectin is used for the diagnosis, monitoring disease activity, treatment guidance and prediction of disease relapse and post-operative recurrence in IBD. There may also potentially be a role for faecal calprotectin in the management of infectious gastroenteritis, acute appendicitis, peptic ulcer disease, cystic fibrosis, coeliac disease, transplant rejection and graft versus host disease.
Although fecal calprotectin is a very sensitive marker for inflammation in the gastrointestinal tract, it is not a specific marker for IBD. Increased levels are also seen in gastrointestinal malignancies, infections, polyps and with the use of nonsteroidal anti-inflammatory drugs. The sensitivity of fecal calprotectin is 95% and specificity is 93%.
Faecal calprotectin levels may vary with age. Children aged from 1 to 4 years old have lower faecal calprotectin concentrations compared with healthy infants (<1 year), and higher faecal calprotectin concentrations when compared with children older than 4 years and adults. Faecal calprotectin may also have considerable day-to-day variation.
Calprotectin assay should be done 2-3 times/year to check the trend of the faecal Calprotectin concentration. The decrease of Calprotectin values down to normal levels in a short period of time is usually an indication of better prognosis.
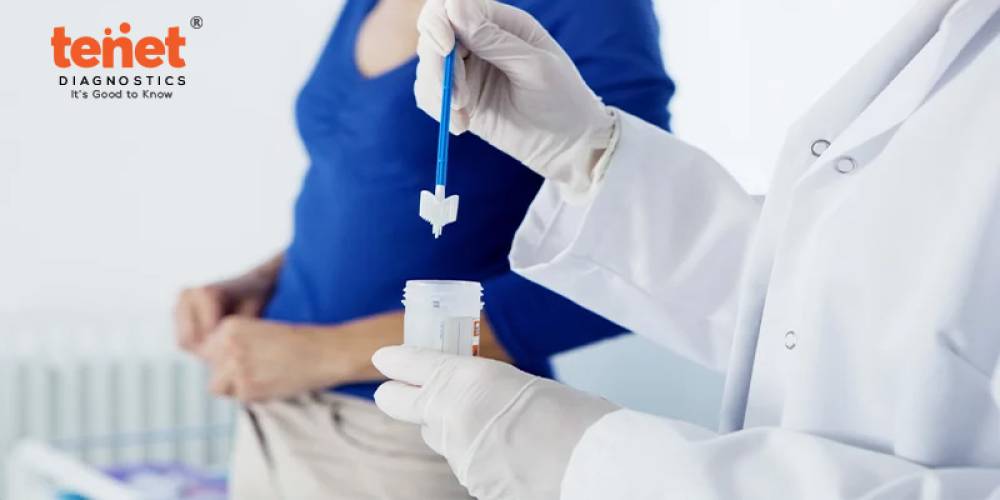
Submit 5 g of first morning faeces sample, collected in a sterile container collected from TENET. Avoid NSAIDs for 3 days before collecting and submitting the sample to laboratory. Get results in 3 days.